Neuroscientists from the University of Pittsburgh, USA, have developed a blood test to detect tau protein build-up in the brain commonly seen in Alzheimer’s. This test eliminates the need for a brain scan or cerebrospinal fluid (CSF) extraction. The study was published in Brain.
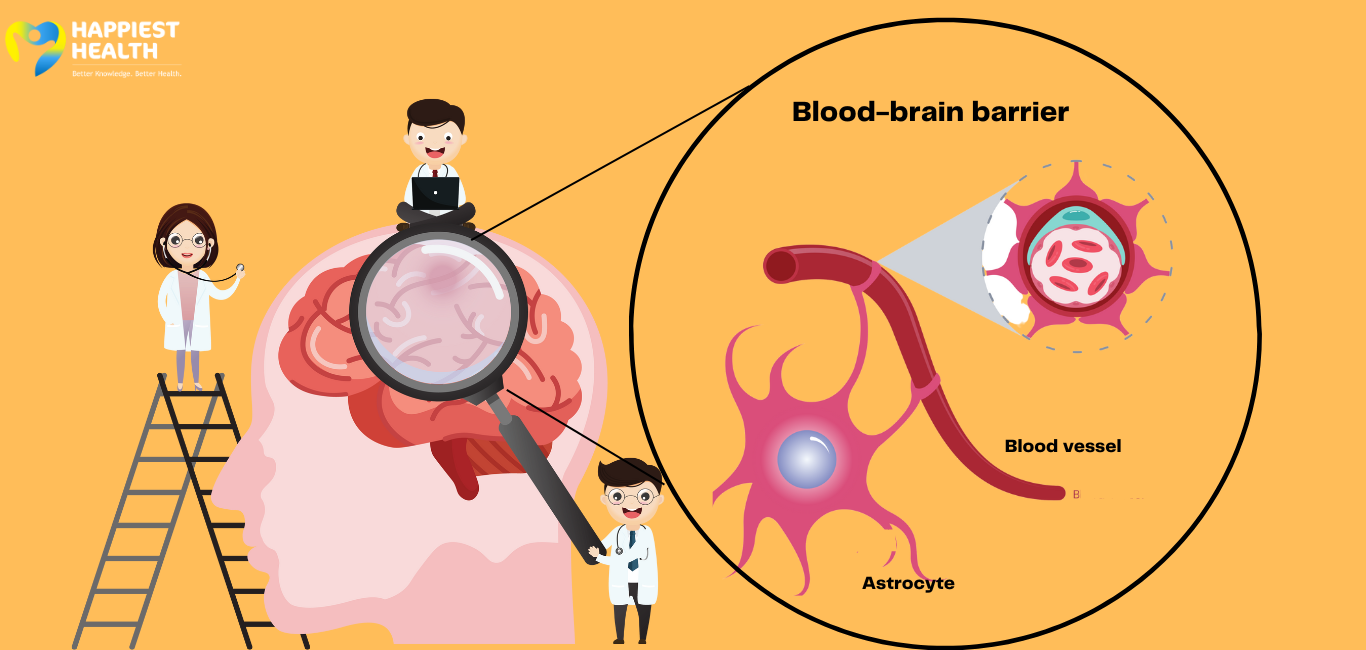
Diagnosis of Alzheimer’s calls for the assessment of the ATN framework: accumulation of amyloid-beta protein (A), tau-tangle formation (T), and loss of neurons due to progressive neurodegeneration (N). Currently, a combination of brain imaging, laboratory testing and CSF extraction are required to assess for Alzheimer’s.
“At present, diagnosing Alzheimer’s disease requires neuroimaging that is expensive and takes a long time to schedule,” Thomas Karikari, assistant professor at the University of Pittsburgh, said in a statement. A blood test can detect the amyloid beta and tau specific to Alzheimer’s. However, assessing nerve cell damage involves CSF extraction, a painful procedure.
Karikari’s team developed an antibody that specifically binds to brain-derived tau (BD-tau) and is detectable in a blood sample. “A blood test is cheaper, safer, and easier to administer, and it can improve clinical confidence in diagnosing Alzheimer’s and selecting participants for clinical trial and disease monitoring,” says Karikari.
The researchers analysed the blood samples of 600 participants in different stages of Alzheimer’s as well as healthy individuals to validate the results of their method. They then compared the BD-tau value obtained from their method with those obtained through conventional CSF extraction and found the results matched. The new blood test could help in differentiating Alzheimer’s from other neurodegenerative conditions, their statement said.
“The development of simple tools detecting the signs of Alzheimer’s in the blood without compromising quality is an important step toward improved accessibility,” Karikari said.
Karikari’s team plans to conduct the validation test on a large-scale population encompassing different racial and ethnic backgrounds.
“While the exact costs are difficult to estimate, it is certain that they will be much lower than the cost of an MRI scan or a spinal tap. If approved [by the FDA] the tests can become available in as early as 2 years,” says Karikari to Happiest Health.