Have you ever met someone for the first time and had a ‘gut’ feeling that they would be your friend? It turns out that the gut microbiome—the little residents of our tummies that help with digestion—plays a role in our social behaviour.
Recent studies published in PLOS Biology and BMC Genomics have shown a strong connection between the gut and socialising. Experiments with zebrafish revealed that gut microbes in young zebrafish prune the ‘social neurons’ in the brain.
Stripe-ing down social behaviour with zebrafish
“Though social behaviour is significantly more complex in humans than in zebrafish, they perform many of the same social behaviours, including aggression, mating, and of particular interest in this study, social approach and orienting. This makes zebrafish an excellent model for understanding how social behaviour develops,” says Dr Judith Eisen, a co-author of both studies.
Zebrafish are widely used in animal studies due to their transparent bodies during their growth. This enables easy observation of changes to structures. Furthermore, they share common genetic features with other higher-order animals. They are also social animals with genes and brain circuits that control social behaviour, similar to those seen in humans, making them a great model to study neural networking.
“Leveraging the strong genetic and neurodevelopmental strengths of the zebrafish system, there have been studies in the field where specific aspects of autism-like behaviour, particularly in the domain of sociability, have been modelled in zebrafish. Zebrafish mutants lacking or having impaired function of genes, that have been implicated in the etiology of autism-spectrum disorder, are being employed in the field,” says Dr Jatin Nagpal from the University College of Cork, Ireland.
Autistic gut-wiring
Previous studies have shown a two-way connection between gut microorganisms and the brain. For example, microorganisms commonly affect mood and stress, but the brain also affects gut microbiota’s digestive and absorption activities.
Research is increasingly demonstrating that the microbiome influences microglia – the immune cells, or ‘cleaners’ of the brain.
According to Dr Nagpal, the microbiome interacts with the nervous system through multiple channels, such as the immune system, metabolite and neurotransmitter secretions, the vagus nerve, and the enteric nervous system. These channels work together to facilitate communication between the microbiome and the brain.
The microglia have two main functions: pruning the nerve cells and protecting them from pathogens. This large population of housekeeping cells constantly survey the brain and keep it clean.
Read: Secret allies: the brain and the immune system
Read: Study finds cells nibble synapses to improve memory
Signs of autism spectrum disorder appear within the first two years of a child’s development. During the studies, Dr Eisen’s team observed the zebrafish during their early developmental stages, a sensitive period for growth. This study reveals new neural pathways form in crucial early development, which could improve our understanding of autism.
Richa Srivastav, a nutritionist from Noida who works specifically on the gut-brain link of autism, says “Whenever there is a dysbiosis (an imbalance of gut microbes) in the gut, there is an alteration in the response of neurotransmitter secretion. Once the gut is corrected, balance is restored.”
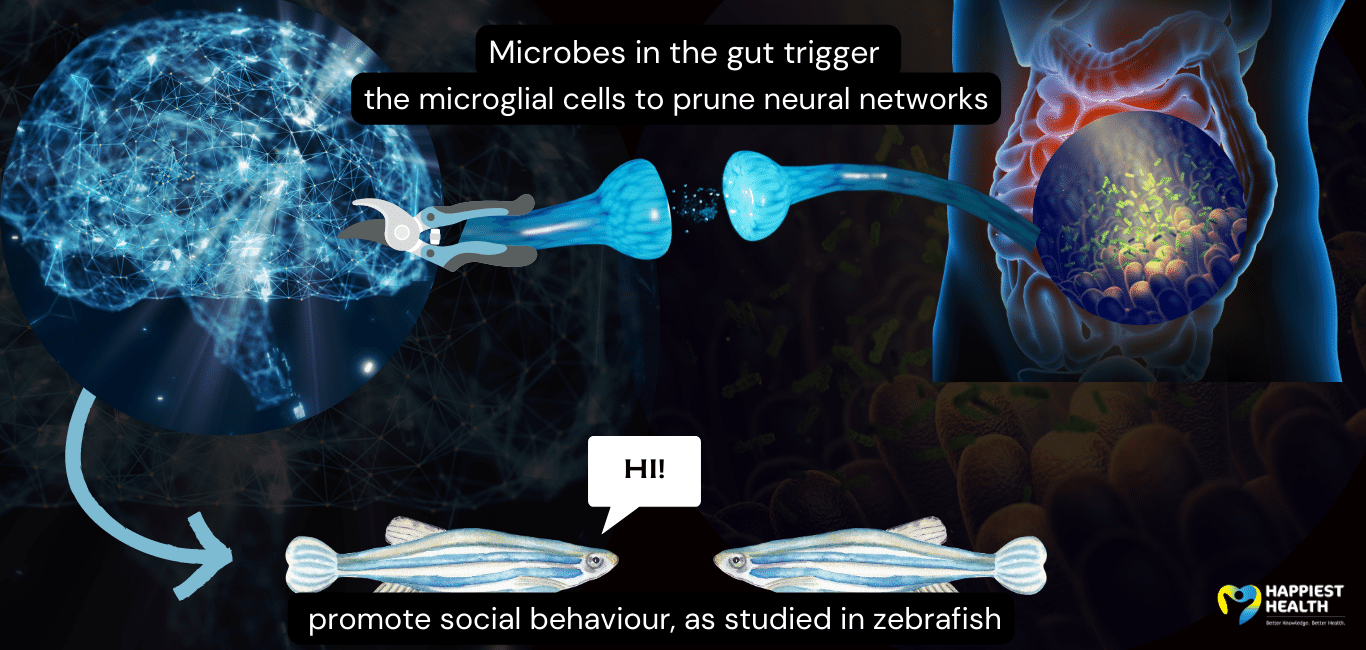
Pruning the networks with microbes
The researchers of the study observed that the zebrafish lacking gut microbiota did not interact with other zebrafish in the neighbouring tank, whereas those with gut microbiota swam to the edge to communicate.
The microglia are linked to the gut microbiome through the complement signalling pathway – an innate immune system mechanism. The microbiota activates the microglia in the brain by releasing chemicals that switch on this immune system.
“We focused on how the microbiota is important for a molecular pathway, called complement signalling, that microglia use to remodel neurons,” says Dr Eisen.
The team found that the gut and microglia together ‘prune’ the neural network involved in socialising.
Srivastav says that synaptic pruning happens in everyone, and a two-year-old has more synaptic connections. Inefficient pruning of these connections can cause behavioural differences in autistic children.
Drawing the path ahead
“The earlier children are given the right nutrition to correct the gut, the better. I have worked with children aged 1 to 1.5 years. Most cases of autism are identified by the age of 3 or 4. However, the younger, the better,” says Srivastav.
The next step of the research is to investigate how the gut microbiome interacts with the host nervous system. Especially the influence of a single bacteria or metabolite on behaviour. “If future studies can narrow down the effects we describe to a particular bacterial strain or molecule, then subsequent work could investigate how that strain or molecule affects humans,” says Dr Eisen.
However, in Dr Nagpal’s opinion, knowing the cause for change would be more helpful. “The future studies would involve digging into mechanisms – to understand how and what specific features of the complex microbiome and its constituent members communicate with the host nervous system to influence its structure-function and emergent behaviour,” he says.