A breakthrough bioink developed recently by researchers at the Terasaki Institute for Biomedical Innovation (TIBI) in Los Angeles, US, may hold the key to bioprinting grafts of functional skeletal muscle tissues in the lab.
The bioink could enable skeletal muscle grafts to be grown or bioprinted, removing the need for muscle transplants from other sites of body. This has the potential to improve how we treat traumatic injuries and muscle disorders, something that currently requires grafting healthy tissue from other parts of the body.
3D bioprinting muscles
Being able to grow (or print) muscle grafts could significantly improve the treatment of traumatic injuries and muscle disorders, which currently require grafting healthy tissue from other parts of an individual’s body.
The researchers did this by developing a new type of bioink to 3D bioprint skeletal muscle tissues, which can be used to treat muscle injuries and disorders.
Researchers at TIBI developed a bioink with microparticles specifically designed for the sustained release of a molecule called IGF-1 or insulin-like growth factor-1. IGF-1 increases the formation and alignment of mature muscle tissue from muscle precursor cells.
The new approach improves overall muscle regeneration, with potential to treat individuals facing muscle loss from traumatic injury or other conditions.
How a loss of skeletal muscle impairs you
Skeletal muscle loss can happen due to certain traumatic injuries, health conditions, or surgical procedures. The muscles of the affected area may lose their function and cause the person problems such as difficulty walking or lifting heavy objects, risk of falling or difficulty with day-to-day work.
Current management strategies for muscle loss involve transplanting healthy muscles from unaffected parts of the body. However, there is a substantial loss of healthy tissue at the donor site leading to impairment of function there.
Transplanted muscle does not have proper nerve connections or new blood vessel connections, which, along with other medical complications, makes full recovery of muscle function difficult.
Challenge of bioengineering muscles
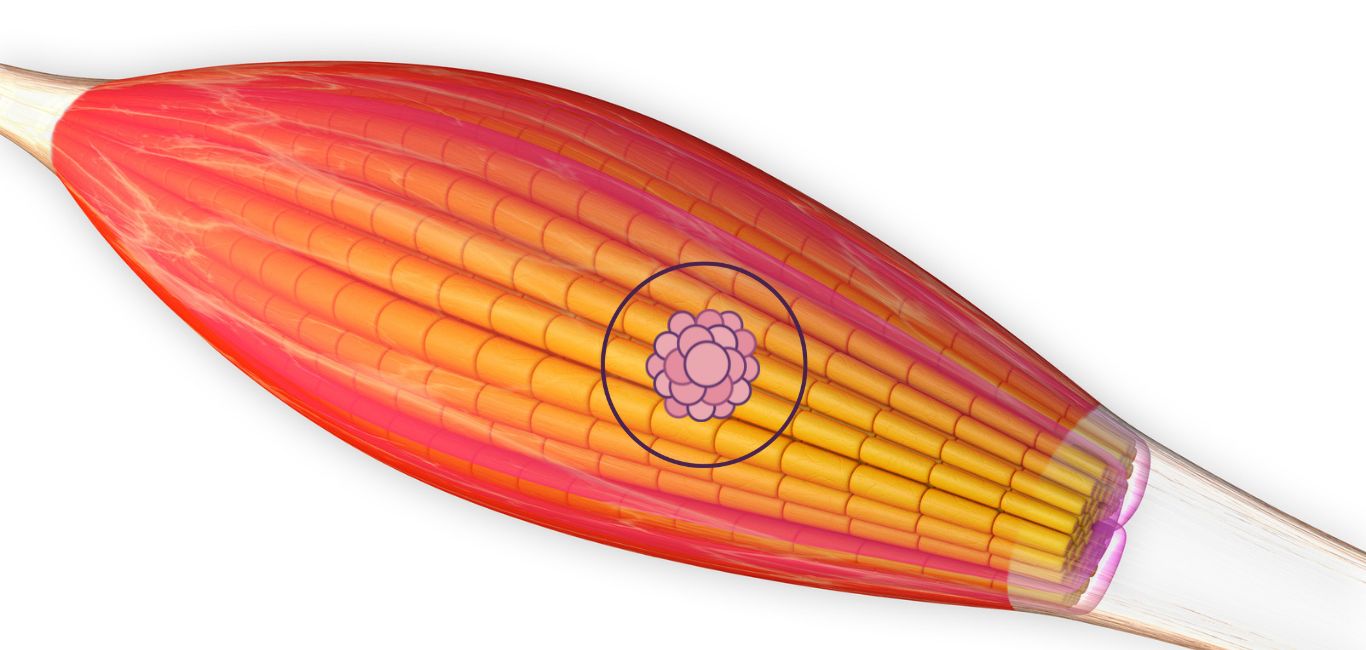
Developing muscles is a slow and gradual process. It starts with round-shaped muscle precursor cells called myoblasts, which multiply and fuse to form long mature myotubes. These tubes transform into muscle fibres — but they need to be properly aligned and oriented for full function.
Current techniques to engineer muscle tissue largely rely on building scaffolds to support existing tissue structures. However, they lack the ability to either form well aligned muscle tissue structures or create fully functional mature muscle tissues.
The growing accessibility of 3D bioprinting is proving to be a promising option for muscle regeneration. The use of bioinks can promote cell survival, printability and tissue formation.
A new bioink to the rescue
TIBI’s bioink can provide sustained release of a specific growth factor involved in tissue repair to fabricate functional muscle tissues. IGF-1 or the insulin-like growth factor-1 plays a central role in muscle repair and modulation of muscle size during regeneration.
“The sustained release of IGF-1 facilitates the maturation and alignment of muscle cells, a crucial step in muscle tissue repair and regeneration,” says TIBI’s director and CEO, Dr Ali Khademhosseini, in a statement.
The 3D bioprinting of skeletal muscle was done using a bioink composed of:
- Gelatin methacryloyl or GelMA (a biocompatible gelatin-based hydrogel)
- Myoblast cells
- Microparticles fabricated for sustained delivery of IGF-1
Earlier studies found that while IGF-1 helps muscles heal and grow, it needs to be present for at least 10 days. TIBI’s study found that the IGF-1 release from coated particles could be sustained for up to 21 days.
In just after a week, the researchers noticed that young muscle cells (myoblasts) turned into longer structures (myotubes), and after 10 days, these muscle structures started contracting and working. It is like giving the building blocks for muscle repair and seeing them work within a certain time frame.
Implanted muscle work in mice
To study the efficacy of the technique, the study also looked at implanting the bioprinted tissues in mice with experimentally induced injuries.
After six weeks, the mice displayed a high degree of muscle tissue regeneration. IGF-1 also induced a well-regulated inflammatory response, another factor that is good for tissue repair.
“We also observed indications of recovery of muscle function in the mice, including their ability to move freely and even jump into their cages,” Dr Nathan Roberto de Barros, first author of the study, tells Happiest Health.
A more comprehensive study is needed completely assess the recovery of muscle function after implantation of muscle constructs, he adds.
Future applications
“There is great potential for using this strategy for the therapeutic creation of functional, contractile muscle tissue,” Dr Khademhosseini says in his statement.
The technique may find applications in regenerative medicine, such as treating muscle injuries and disorders, adds Dr Barros. Additionally, it could be explored for repairing or enhancing other tissues and organs beyond muscle, such as skin, cartilage, and potentially more complex structures, he elaborates.
“The translation of this research into clinical applications can vary widely from several years to decades and depends on several factors, including further research, regulatory approvals and clinical trials,” says Dr Barros.
Read More: Hyderabad Scientists give India a head start in race to develop 3D printed cornea